Chemistry Waec Practical Answers 2024
(3a)
(i)ammonia (NH₃)
(ii)carbon dioxide (CO₂).
(3b)
(i)A graduated pipette
(ii) A burette can be used.
(3c)
A fume cupboard in a laboratory is used to provide ventilation that limits exposure to toxic fumes, vapors, or dust.
*2024 WAEC CHEMISTRY PRACTICAL ANSWERS*
*NUMBER THREE*
(3a)
(PICK TWO)
(i ) Carbon dioxide (CO2)
(ii) Ammonia (NH3)
(iii) Oxygen (O2)
(iv)Hydrogen chloride (HCl)
(3b)
(PICK TWO)
(ii) Burette
(iii) Volumetric flask
(iv) Micropipette
(3c)
Safely contain and remove harmful fumes, gases, or vapors generated during experiments, preventing their exposure to lab personnel.
(3d)
(PICK THREE)
(i) Safety goggles/glasses
(ii) Lab coat/apron
(iii) Gloves
(iv)Face mask/respirator
(v) Closed-toe shoes/footwear
(vi) Ear protection (if needed for specific experiments or environments)
(3d)
(i)safety goggles.
(ii)lab coats.
(iii)gloves.

QUESTION 1
(1a)
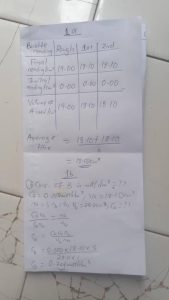
TABULATE PLEASE
Burette | Rough| 1st | 2nd
Final reading(cm³) | 19.00 | 18.10| 18.10|
Initial reading(cm³) | 0.00| 0.00| 0.00
Volume of A used(cm³) | 19.00| 18.10| 18.10|
Average titre = 18.10+18.10/2
= 18.10cm³
(1bi)
Conc. of B in mol/dm³ = ??
Ca = 0.200mol/dm³, Va = 18.10cm³
Na= 1, Nb = 5, Vb= 25.00cm³ , Cb= ??
CaVa/CbVb= Na/Nb
Cb = CaVaNa/VbNb
Cb= (0.200*18.10*5)/(25.0*1)
Cb= 0.724mol/dm³
(1bii)
Con. of B in g/dm³ = ??
Con.(g/dm³) = Mass*1000/volume
= 3.8*1000/250
= 15.2g/dm³
(1biii)
FeSO₄<—> Fe²⁺ + SO₄²⁻
1 mol of FeSO₄ 1mol Fe²⁺
0.724mol/dm³ xmol/dm Fe²⁺
x= 1*0.724 = 0.724mol/dm³
Mole= Con.*Vol/1000
Mole = 0.724*250/1000
Mole= 0.181mol
Mass= mole * m.m
= 0.181*56
= 10.136g
(1biv)
Percentage= 10.136*100/15.2
= 66.7%
======================================================
QUESTION 2
(2a)
When all of C is added to a test tube with about 10cm³ of distilled water and shaken, the mixture is filtered. The residue and the filtrate are obtained.
Observations:
The residue: This will contain the insoluble salt(s) present in C.
The filtrate: This will contain the soluble salt(s) present in C.
(2bi)
Heating about 2cm³ of the filtrate in a boiling tube strongly.
Observations:
If there is effervescence (bubbling), it indicates the presence of a carbonate salt in the filtrate.
The gas evolved is likely carbon dioxide (CO₂).
(2bii)
Adding dilute HCl to half of the residue in a test tube.
Observations:
If there is effervescence (bubbling), it indicates the presence of a carbonate salt in the residue.
The gas evolved is likely carbon dioxide (CO₂).
(2biii)
Adding aqueous NaOH in drops, then in excess, to about 2cm³
Observations:
If a white precipitate forms upon adding NaOH drops, and it dissolves upon excess addition of NaOH, it indicates the presence of a metal hydroxide in the solution.
(2biv)
Adding aqueous ammonia in drops, then in excess, to another 2cm³ of the clear solution from (ii).
Observations:
If a colored precipitate forms upon adding ammonia drops, and it dissolves upon excess ammonia addition, it indicates the presence of a metal hydroxide in the solution.
======================================================
QUESTION 3
(3a)
(i)ammonia (NH₃)
(ii)carbon dioxide (CO₂).
(3b)
(i)A graduated pipette
(ii) A burette can be used.
(3c)
A fume cupboard in a laboratory is used to provide ventilation that limits exposure to toxic fumes, vapors, or dust.
(3d)
(i)safety goggles.
(ii)lab coats.
(iii)gloves.
================


Please sir the images are always blur, I will appreciate it sir if you can help us upload clear images.