WAEC CHEMISTRY PRACTICAL ANSWERS 2021
Here is the waec chemistry practical answers for 2021.
Chemistry practical answers
3ai
The titre value would be higher than 23.50cm³.
3aii
This is because when distilled water was added to the base , the base becomes less concentrated , hence it’s easier to titrate than a concentrated base.
3bi no reaction occurs , copper is less electropositive than Zinc , so cannot displace it in the reaction.
ii), KOH is a deliquescent compound, so it would absorb water from the atmosphere to form sticky particle in the watch glass.
III), when few drops of phenolphthalein is dropped in the watch glass , it changes colour to pink because it’s a basic solution (KOH).
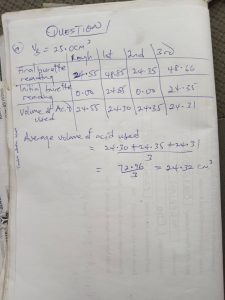
(1a)
Volume of burrete = 50.00cm³
Volume of pipette = 25.00cm³
Indicator = Methyl orange
TABULATE
Final burette readings (cm³); 37.50 | 22.50 | 37.50 | 44.50
Initial burette readings(cm³); 0.00 | 0.00 | 0.00 | 0.00
Volume f Acid used (cm³); 37.50 | 22.50 | 22.50 | 22.50
Volume of A used = 1st + 2nd + 3rd/3
= 22.50+22.50+22.50/3
Average volume of A used = 22.50cm³
(1bi)
Concentration of A ( CA )?
2.03g in 500cm³ of solution
Volume = 500cm³/1000 = 0.500dm³
In g/dm³ = 2.03/0.5 = 4.06g/dm³
To find concentration in mol/dm³
Conc. of A in mol/dm³ = Conc. of A in g/dm³/Molar mass
Conc. of A in mol/dm³ = 4.06/36.5
= 0.1112mol/dm³
Molar mass of A (HCL) = 1+35.5 = 36.5g/mol
(1bii)
Number of mole of the Acid in average titre
Na = CaVa
= 0.1112 × 22.50
= 2.52moles
(1biii)
Nb= ? Na= 3, Ca= 0.1112mol/dm³, Cb = 0.12mol/dm³ , Va = 22.50cm³, Vb = 50.00cm³
CaVa/CbVb= nA/nB
0.1112×22.50/0.12×50 = 3/nB
nB × 2.502 = 18
nB = 18/2.502 =
:. nB = 7.194moles
= 7moles(approx.)
(1biv)
Mole ratio of Acid to base
nA:nB = 7:3



We gat you..


please the answers are not loading 😢 fast sir
Hoping to get questions and answers quickly
Love u so much
Thank you sir
Sorry I never knew there was a reply button
Thanks your works are great 🙌
Thanks
Thanks And GOD Bless u. Amen
Thanks alot am so greatful
Sorry